Boyle’s and charles law gizmo – The Boyle’s and Charles’ Law Gizmo is an engaging and interactive tool that allows users to investigate the relationships between pressure, volume, and temperature in a gas. This powerful tool provides a unique and immersive way to learn about the fundamental principles of gas behavior.
By manipulating the Gizmo’s controls, users can observe firsthand how changes in one variable affect the other two. This hands-on approach deepens understanding and promotes a deeper appreciation for the underlying concepts.
Boyle’s Law

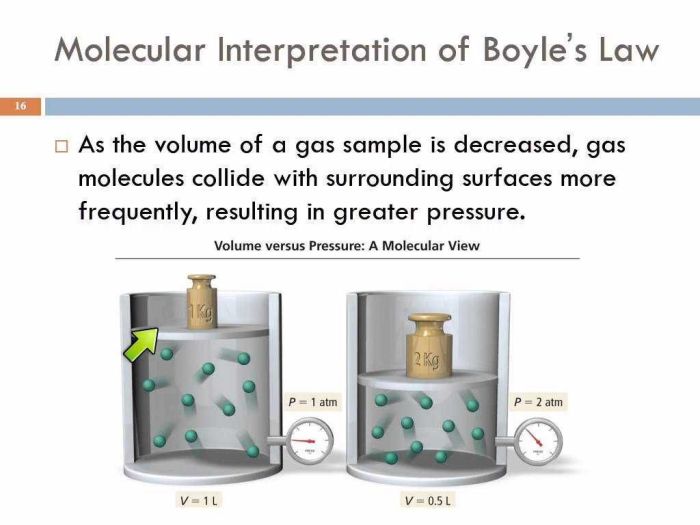
Boyle’s Law describes the inverse relationship between the pressure and volume of a gas at constant temperature. As the pressure on a gas increases, its volume decreases proportionally, and vice versa.
This relationship can be expressed mathematically as:
P₁V₁ = P₂V₂
The Boyle’s and Charles Law Gizmo is a great tool for exploring the relationship between pressure, volume, and temperature of gases. If you’re looking for more practice with these concepts, check out the acid base or salt worksheet . After you’ve mastered these topics, come back to the Boyle’s and Charles Law Gizmo to see how you can apply them to real-world situations.
where:
- P₁ and P₂ are the initial and final pressures of the gas, respectively
- V₁ and V₂ are the initial and final volumes of the gas, respectively
Applications of Boyle’s Law
Boyle’s Law has numerous applications in real-world situations, including:
- Scuba diving:As scuba divers descend deeper into the ocean, the pressure on their bodies increases. Boyle’s Law explains why their lungs shrink in volume at greater depths.
- Pressure cookers:Pressure cookers use Boyle’s Law to create a high-pressure environment inside the cooker. This increased pressure raises the boiling point of water, allowing food to cook faster.
- Air compressors:Air compressors use Boyle’s Law to increase the pressure of air by reducing its volume. This compressed air is then used for various applications, such as powering pneumatic tools.
Limitations of Boyle’s Law
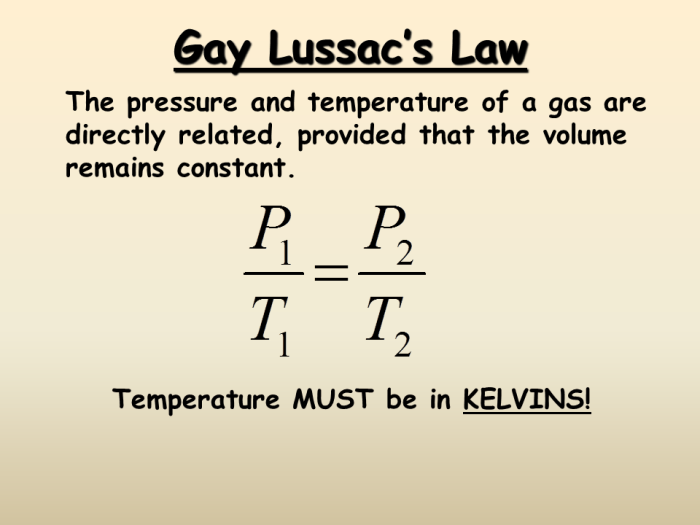
Boyle’s Law assumes that the temperature of the gas remains constant. However, in real-world situations, temperature changes can occur, affecting the accuracy of Boyle’s Law calculations.
Additionally, Boyle’s Law does not apply to gases at very high pressures or very low temperatures, where the behavior of gas molecules deviates from ideal gas behavior.
Charles’ Law
Charles’ Law describes the relationship between temperature and volume in a gas. It states that the volume of a gas is directly proportional to its absolute temperature, assuming constant pressure. In other words, as the temperature of a gas increases, its volume also increases, and vice versa.
Applications of Charles’ Law
- Hot air balloons: As the air inside a hot air balloon is heated, it expands and becomes less dense than the surrounding air, causing the balloon to rise.
- Gas cylinders: Charles’ Law is used to calculate the volume of gas that can be stored in a cylinder at a given temperature.
- Thermometers: Some thermometers use the expansion of a gas (such as mercury or alcohol) to measure temperature.
Limitations of Charles’ Law, Boyle’s and charles law gizmo
- Charles’ Law is only accurate for ideal gases, which do not exist in reality.
- The law does not apply at very high pressures or low temperatures, where the behavior of gases deviates from ideal behavior.
Boyle’s and Charles’ Law Gizmo

Features and Functionality
The Boyle’s and Charles’ Law Gizmo is an interactive simulation that allows students to investigate the relationships between pressure, volume, and temperature in a gas. The Gizmo features a virtual gas sample that can be manipulated by changing the pressure, volume, or temperature.
Students can also add or remove gas molecules from the sample.The Gizmo provides real-time feedback on the changes in the gas sample. Students can observe how the pressure, volume, and temperature change as they manipulate the sample. The Gizmo also includes a graph that shows the relationship between the pressure, volume, and temperature of the gas.
Applications in the Classroom
The Boyle’s and Charles’ Law Gizmo can be used in a variety of ways in the classroom. For example, the Gizmo can be used to:
- Demonstrate the relationships between pressure, volume, and temperature in a gas.
- Investigate the effects of adding or removing gas molecules from a sample.
- Predict the behavior of a gas under different conditions.
- Design experiments to test the relationships between pressure, volume, and temperature in a gas.
The Gizmo is a valuable tool for teaching students about the behavior of gases. It is an engaging and interactive way for students to learn about this important topic.
Real-World Applications of Boyle’s and Charles’ Laws
Boyle’s and Charles’ Laws are fundamental principles in physics and chemistry that describe the behavior of gases under varying conditions. These laws have numerous practical applications across various fields, enabling the design and operation of devices and systems that rely on gas behavior.
Engineering
- Internal Combustion Engines:Boyle’s Law governs the compression of air-fuel mixture in the cylinders, determining the engine’s power and efficiency.
- Refrigeration Systems:Charles’ Law explains the expansion and contraction of refrigerant gases as they undergo phase changes, enabling efficient cooling.
- Scuba Diving:Boyle’s Law is crucial for understanding the pressure changes experienced by divers as they descend and ascend, guiding safe diving practices.
Chemistry
- Gas Chromatography:Boyle’s Law is used to separate and analyze gases based on their different compressibilities.
- Chemical Reactions:Charles’ Law helps predict the volume of gases produced or consumed in chemical reactions, informing reaction stoichiometry.
- Gas Laws Calculations:Boyle’s and Charles’ Laws provide a framework for calculating gas properties such as volume, pressure, and temperature, aiding in experimental design and analysis.
Physics
- Weather Forecasting:Boyle’s Law explains the expansion of air as it rises, leading to pressure changes that influence weather patterns.
- Balloon Flight:Charles’ Law governs the expansion of helium or hot air in balloons, enabling controlled flight.
- Vacuum Technology:Boyle’s Law is applied in vacuum pumps to create low-pressure environments for various industrial and scientific applications.
Emerging Technologies
- Fuel Cells:Boyle’s Law is crucial for optimizing the efficiency of fuel cells, which convert chemical energy into electricity.
- Membranes for Gas Separation:Charles’ Law guides the design of membranes that selectively allow certain gases to pass through, enabling efficient gas purification.
- Aerospace Engineering:Boyle’s and Charles’ Laws are used in spacecraft design to control cabin pressure and manage gas flow in propulsion systems.
Essential FAQs: Boyle’s And Charles Law Gizmo
What is the Boyle’s Law Gizmo?
The Boyle’s Law Gizmo is an interactive simulation that allows users to explore the relationship between pressure and volume in a gas.
What is the Charles’ Law Gizmo?
The Charles’ Law Gizmo is an interactive simulation that allows users to explore the relationship between temperature and volume in a gas.
How can I use the Boyle’s and Charles’ Law Gizmo in my classroom?
The Boyle’s and Charles’ Law Gizmo can be used in a variety of ways in the classroom, including as a demonstration tool, a hands-on activity, or a formative assessment.